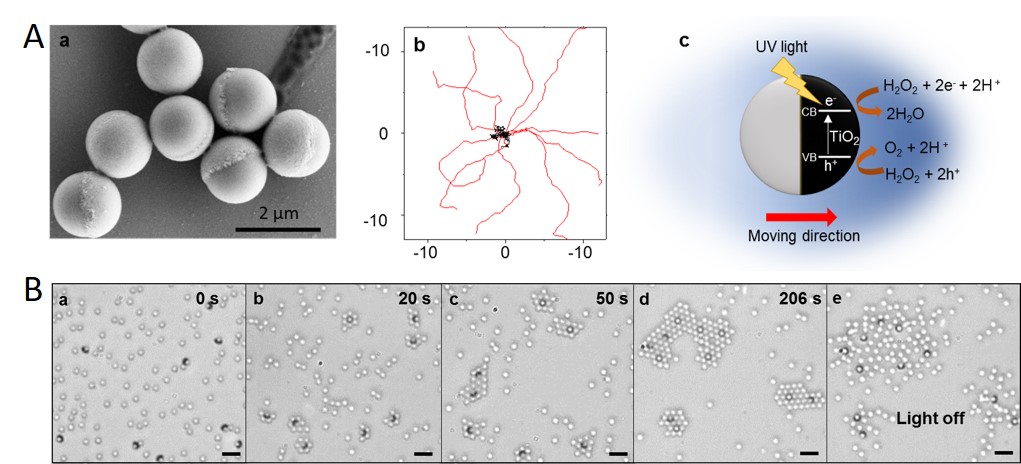
Figure: Top A: Light-activated self-propelled Janus particles. a) SEM image of light activated Janus particles showing the TiO2 cap upon SiO2 sphere. b) The UV mediated catalytic decomposition of H2O2 at the TiO2 face of the particle. The gradient causes the particle to move by diffusiophoresis towards the TiO2 surface. The units in (b) are µm. c) Trajectories of active Janus particles when the UV light is on (red) and off (black) over 3s acquisition. Bottom panel B: Light-controlled crystal formation in active-passive colloids (under light illumination ). (a-d) A sequence of images showing the growth of crystals under UV illumination. Active particles can be distinguished from the passive ones by the dark crescent or ring at their edge. (e) Crystals melt by thermal diffusion when the light is turned off. Scale bar indicates 5 µm.
Chemical motors
Dhruv P. Singh, Udit Choudhury, Tingting Yu, Mariana Alarcon-Correa, Mihail Popescu, Peer Fischer
We study out-of-equilibrium dynamic assembly and self-organization with chemically active colloids, which are also known as chemical motors. Apart from dynamic assembly, these self-propelling colloids also show collective effects similar to those observed in living systems. A major challenge is to obtain sufficiently large numbers of active colloids and to operate them stably at high density. Our research addresses these questions. Very recently, we could show that self-propelling colloidal clusters can even form due to spontaneous symmetry breaking [1]. We could also show that we can realize a material from active colloids whose viscosity can be switched optically [2].
Janus particles composed of SiO2 and TiO2 halves self-propel when illuminated with UV light (see Figure Aa-b). The propulsion arises from the photocatalytic decomposition of hydrogen peroxide at the TiO2 surface by UV- promoted electron hole pairs (see Figure Ac). The reaction products develop near the TiO2 hemisphere and thus induce a chemical gradient between the poles of the Janus colloid, leading to self-phoretic motion of the particle. The chemical gradient can also induce attractive phoretic interactions between the colloids leading to the formation of the assemblies or structures. When passive SiO2 colloids are added, the active colloids induce the assembly of the passive colloids. Small clusters are formed that are themselves mobile (Figure Ba-d). The entire self-assembly is controllable and reversible through the UV illumination: when the light is switched off (Figure Be), the clusters “melt”.
Chemical motors show rich behaviours and serve as model systems for active matter. Dynamic self-assembly leads to structures that cannot be obtained in purely passive systems [3].
Literature:
[1] T. Yu, P. Chuphal, S. Thakur, S.Y. Reigh, D.P. Singh, P. Fischer, Chem. Comm., 54, 11933-11936, 2018.
[2] Udit Choudhury, Dhruv P. Singh, Tian Qiu, Peer Fischer, Adv. Mat., (1807382), 2019.
[3] Dhruv P. Singh, Udit Choudhury, Peer Fischer, Andrew G. Mark, Adv. Mat. 29, 1701328, 2017.
